|
Development of microfluidic organ-on-chip systems with rapid temperature control for optical nanoscopy |
|
Project 7 - Matteo Boninsegna Cherry Biotech |
|
Objectives: (1) Development of microfluidics organ-on-chip platforms that allow for the maintenance and support of organ functions by perfusing tissue sections while facilitating high speed, high resolution imaging of cellular morphology (2) Implementation of rapid temperature changes through steering of fluid flow facilitates short-term very high resolution imaging and deceleration of physiological function (3) Nanoscopy of fenestration dynamics in perfused organ slices (4) Control of gas partial pressure will enable the control and simulation of physiological stress, such as hypoxia |
|
Expected Results: (1) Perfusion of liver slices and maintenance of physiological function demonstrated for several hours (2) rapid fluid exchange with temperature control demonstrated in support of high resolution 3D nanoscopy (3) Controlling gas partial pressure while maintaining SR-SIM measurements demonstrated (4) imaging of fenestration dynamics in intact liver sinusoids demonstrated |
|
Liver sinusoidal endothelial cells (LSECs) form particular vessels, called sinusoids, within liver parenchyma and express nanopores on their surface. LSECs also express nanopores on their surface. These nanopores, known as fenestrations, allow the passage of molecules from blood stream to hepatocytes and vice versa. Unfortunately, fenestrations are so small that cannot be observed with common microscopy techniques. Moreover, it is very difficult to culture LSECs in vitro, but also to maintain cut liver sections, without losing their phenotype after few hours. Main aim of DeLIVER project is to shed light on the behavior of fenestrations by implementing high throughput super resolution microscopy (nanoscopy) and organ on a chip technology. |
|
Project Lead: Early Stage Researcher:
|
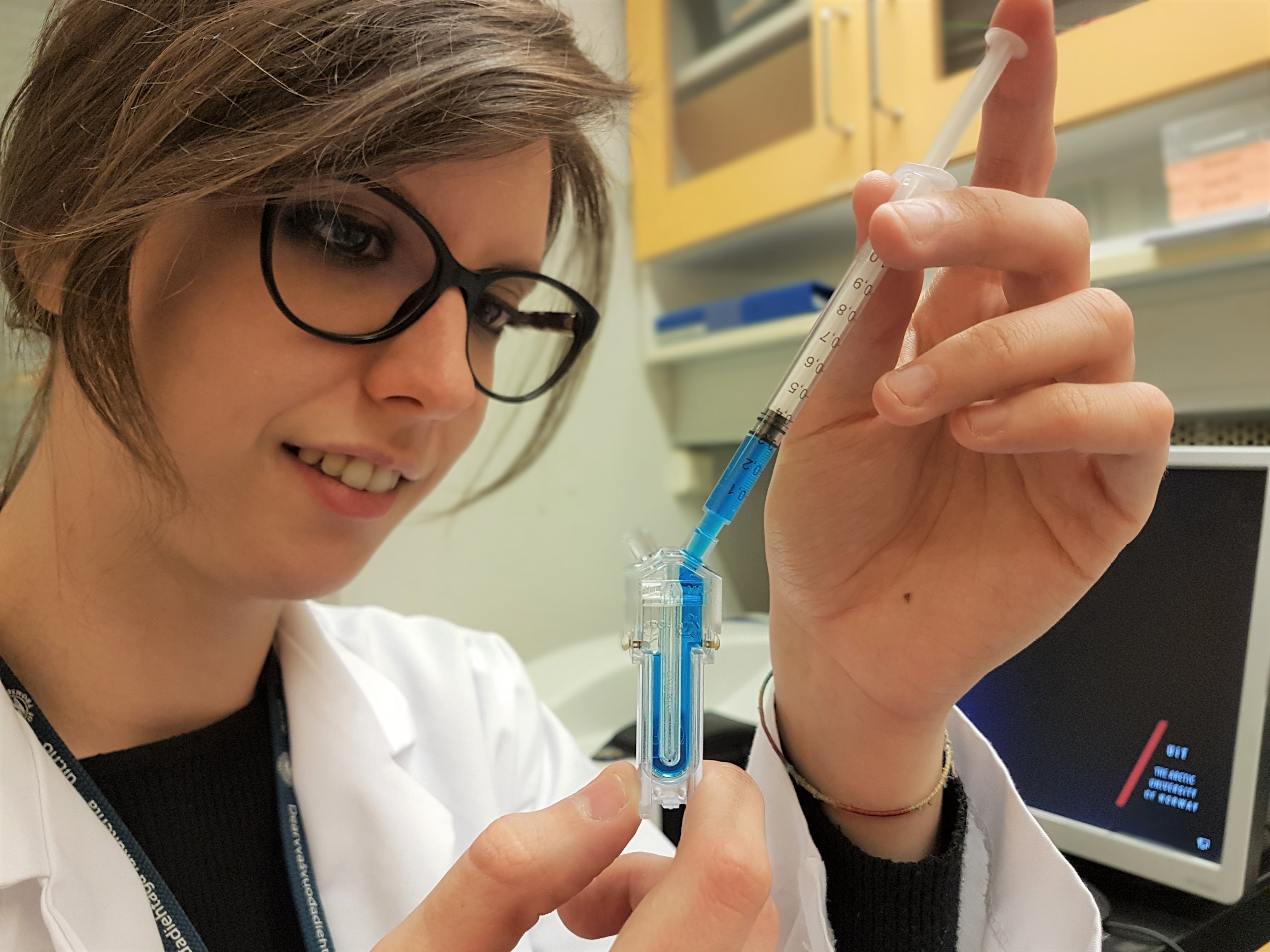
I am part of the DeLIVER project as industrial PhD-student. Particularly, I work at CherryBiotech (Rennes, France) which is a microfluidics and organ on a chip startup. Inside Cherry’s R&D team I am developing systems that control different aspects of the cell microenvironment such as temperature, perfusion and much more. Thus, my role is to provide other early stage researchers (ESRs) with devices for LSECs culturing and liver slices maintaining. Comparing to traditional cell culture methods, these platforms will better recapitulate the physiology of liver sinusoids. Furthermore, to carry out fenestration live imaging, the devices will be compatible with ultrafast optical nanoscopes.
Figure 1: One of our last disposable and various control units.
Figure 2: Recent picture of the CherryBiotech team in Lannion (Brittany, France).
|
|
Extending the versatility of optical nanoscopy by 3D optical force spectroscopy |
|
Project 6 - Zofia Maria Korczak University of Gothenburg |
|
Objectives: (1) Development of 3D optical force spectroscopy of endothelial cells by calibration of polymer bead deviations by holographic optical tweezers near surfaces (2) Integration of the force spectroscopy system with microfluidics and nanoscopy to monitor cellular response in vitro (3) Determine changes in cellular fenestrations and the morphology of sieve plates in response to locally exerted forces (4) Local enrichment of nanoparticle preparations by optical tweezers and cellular response in perfused liver sections |
|
Expected Results: (1) 3D optical force spectroscopy apparatus operational and calibrated (2) mechanical properties of healthy, living LSEC membranes determined (3) integration of nanoscopy with force spectroscopy system completed (4) response of fenestrations and sieve plates to local, external forces determined (5) changes in membrane elasticity due to exposure of LSECs to external agents and their reversibility determined in microfluidic cell culture |
|
Project Lead: Early Stage Researcher:
|
My name is Zofia Korczak and I am a physicist originating from Poland. I was awarded both my Bachelor’s degree (Nanotechnology) as well as my Master’s degree (Molecular Biophysics) from the Adam Mickiewicz University in Poznań, Poland. During my later studies I specialized in photobiophysics because I wished to continue my academic career path using physical approaches (mostly taking advantage of optical techniques) for biological problems. Before starting the PhD studies in Sweden, I worked for one year as a research assistant in the Institute of Micro and Nanotechnology in Madrid. My work there was focused on investigation of ionic liquid droplets, more exactly how to place them and how to move them along silicon nanowires. My project within this ITN consortium is to extend the versatility of optical nanoscopy by 3D force measurements, which means that I will investigate properties of fenestrations in endothelial liver cells using holographic optical tweezers. By measuring small changes in applied forces one can determine and characterize e.g. cells’ membrane elasticities. This technique allows for comparison studies of cells and tissue with different phenotypes and, furthermore, the possibility of using multiple optical traps simultaneously gives us a wide range of potential geometries to perform such experiments. |
|
High speed optical nanoscopy for the optimization of vesicle uptake by liver cells |
|
Project 5 - Jennifer Cauzzo University of Tromsø - The Arctic University of Norway |
|
Objectives: (1) real time nanoscopy study of LSEC-mediated uptake of vesicles (2) determination of intracellular fate and optimization of vesicle properties (3) determination of the factors affecting uptake, particularly gender, age and liver impairment (4) production of nano vectors for distribution across the network |
|
Expected Results: (1) Clear understanding of the fate of nano delivery systems and associated drugs in LSEC established (2) uptake with respect to bioavailability of vector-associated drugs optimized (3) toxicity and safety of nano delivery systems and their clearance in liver established (4) effects of gender, age and liver impairment on nano vector uptake, first step to individualized drug therapy established (5) effects on the morphology and function of isolated LSEC determined |
|
Project Lead: Early Stage Researcher:
JENNIFER CAUZZO PhD student, pharmacist Italian in Norway | 21/05/1993 +47 930 19 712 | +39 349 3099100 This email address is being protected from spambots. You need JavaScript enabled to view it. www.linkedin.com/in/jennifercauzzo |
Born and raised in Italy, Jennifer took a 5-years single cycle degree as a combined Bachelor and Master in pharmacy at the University of Padova (Italy). Working in a hospital pharmacy for few months got her closer to the field of pharmaceutical technology that she then chose as major. She did her experimental thesis as an Erasmus+ trainee at UiT The Arctic University of Norway, where she analyzed the physical chemistry of complexation equilibria with cyclodextrins. She obtained the state qualification for the profession of pharmacist and started her PhD project in nanomedicine with the Drug Transport and Delivery Research Group in Tromsø. As the pharmacist delivers medicines to patients, she is now working to deliver molecules of interest to the liver. She operates with lipid-based nanosystems, mainly liposomes, with the aim of investigating their interactions with the surroundings. First interest is the full characterization of marker-containing formulations and the comparison of technological outcomes (e.g. size, zeta-potential, dye-lipid interaction and stability) for different production methods. At the same time, much of her focus is on visualizing the fate of nanocarriers when in contact with cells. Her final goal would be following the internalization and intracellular pathways of her nanovectors in real-time nanoscopy.
|
|
Development of 3D microfluidic cultivation and cell manipulation systems for optical nanoscopy |
|
Project 4 - Alessandra Dellaquila Elvesys Innovation Center, France |
|
Objectives: (1) Development of microfluidics platforms integrated with high-speed SR-SIM (2) Enabling controlled exposure of endothelial cells to small molecules and lipid nanoparticles while facilitating high speed, high resolution imaging of cellular morphology (3) Extension of microfluidics to facilitate LSECs endothelial tube formation replicating sinusoids in vitro in 3D cell culture and efficient fluorescent staining (4) Nanoscopy of fenestration dynamics in endothelial tube system |
|
Expected Results: (1) High resolution imaging of LSEC dynamics in microfluidics system in vitro performed (2) small-molecule interactions with LSECs at varying concentrations assessed (3) high speed nanoscopy of LSECs within 3D endothelial tubes |
|
Project Lead: Early Stage Researcher:
|
Alessandra Dellaquila is from Torino, Italy. She completed her Bachelor and Master of Science (2011-2016) in Biomedical Engineering at Politecnico di Torino. For her Master thesis, she worked on statistical characterization of surface wettability of polydimethylsiloxane in the Laboratory of Nanotechnology for Precision Medicine (Italian Institute of Technology, Italy). In 2017 she started working as Research Fellow at the Italian National Research Center (ISTEC-CNR) on advanced nanocomposite biomaterials for tissue engineering. Since July 2018, she is a PhD student at the Faculty of Physics, Bielefeld University (Germany), under the supervision of Prof. Thomas Huser. Her research, based in Paris at Elvesys Innovation Center, deals with development of microfluidic platforms integrated with super-resolution microscopy for 3D human liver biology.
|
Page 3 of 4