|
Assessing the histological diversity of liver sinusoidal endothelial cells by optical nanoscopy |
|
Project 9 - Lianne van Os Vrije Universiteit Brussel |
|
Objectives: (1) SR-SIM assessment of the morphology and diversity of LSECs in different liver diseases (e.g. cancer, autoimmune disease, alcoholic and non-alcoholic fatty liver disease) by isolating cells from human donor material and mouse models of liver injury (e.g. liver fibrosis, non-alcoholic fatty liver disease, cancer) (2) assess LSEC morphology and their interactions with other liver cells such as hepatic stellate cells and hepatocytes based on high-resolution imaging techniques (nanoscopy, EM) using immunohistochemistry of liver tissue and 3D hepatic spheroid cultures (3) heterogeneity of LSECs isolated from diseased liver tissue assessed by correlative molecular analysis techniques and SR-SIM with extended z-resolution |
|
Expected Results: (1) LSECS from human and/or mouse pathological liver specimens isolated, fixed, and sorted based on specific liver disease (2) morphology of isolated LSEC samples analyzed by SR-SIM (3) morphology and cellular identity confirmed by SR-SIM within the consortium and correlated to electron microscopy |
|
Globally, liver disease accounts for 2 million deaths per year of which cirrhosis, an advanced stage of fibrosis, causes 1.16 million deaths 1. Fibrosis is a response to chronic liver injury that can be initiated by several factors such as alcohol, non-alcohol fatty liver disease and viral hepatitis 2. LSECs play an important role in fibrosis 3-5and it is even suggested that LSECs could play a role in fibrosis initiation as the their specific phenotype changes (also known as capillarization) before fibrosis occurs in several liver diseases6, 7. LSECs line the sinusoidal wall and are characterized by the absence of a basal lamina and the presence of open pores also known as fenestrae that act as a dynamic filter8. During capillarization LSECs lose their fenestrations and obtain a basement membrane. Research aimed to elucidate mechanism involved in this capilarization of LSECs has focused on LSEC cultured in mono-layers whereby these cells capillarize due to the change to the artificial environment9-11. Co-culture of LSECs with other liver cell types or conditioned medium of other liver cells can improve LSEC function in culture 10, 12. However, to study LSECs in both healthy and diseased conditions, improvements need to be made for in vitroculture of LSECs. Therefore, we aim to develop a 3D spheroid culture with LSECs, hepatocytes and HSCs 13, 14to unravel the morphology and diversity of LSECs in both healthy as diseased states. In this case, the health status of LSECs can be determined by the expression of functional markers and fenestrae assessment by conventional Electron Microscopy (EM) and Super Resolution Microscopy (SRM) that will be developed by our consortium. Diseased states can be induced by for example Acetaminophen (for hepatocyte damage) or Monocrotaline (for LSEC damage) administration. In parallel, to get a complete overview on LSEC physiology in different liver etiologies, LSEC presence and morphology in different mouse models of liver disease will be investigated by immunohistochemistry and SRM of paraffine imbedded liver tissue.
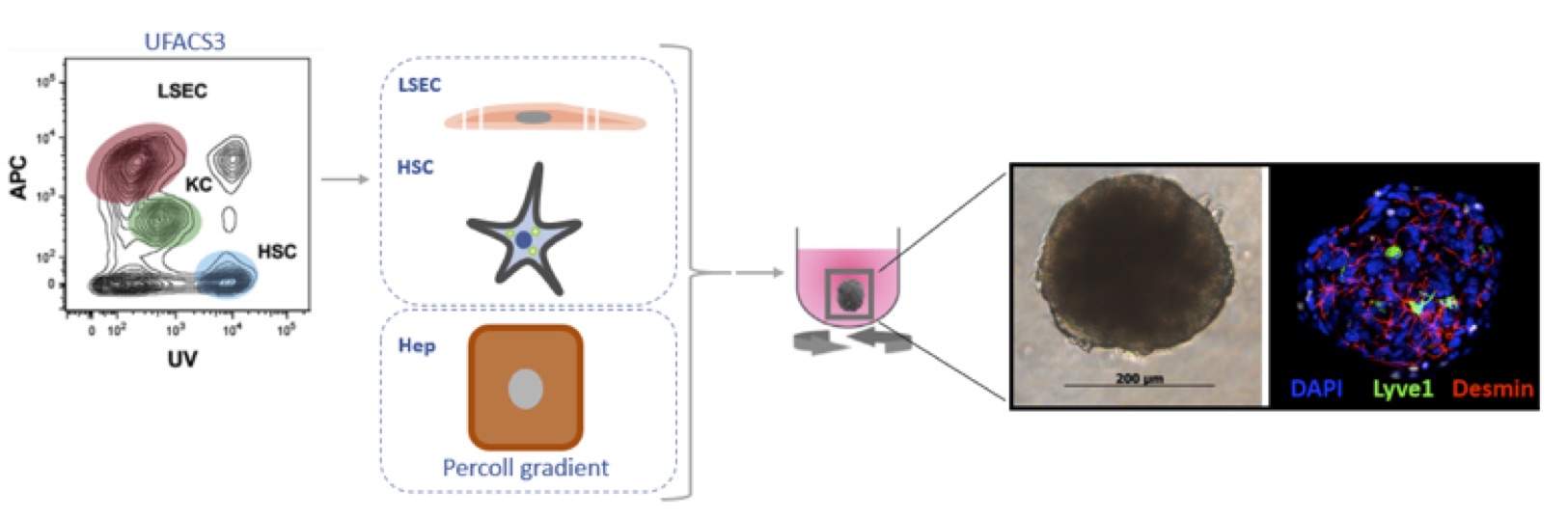
Figure1: Schematic representation of spheroid formation procedure. HSCs and LSECs are isolated via the UFACS314 procedure and hepatocytes by a Percoll gradient. The freshly isolated cells are allowed to form spheroids in 96-well round bottom plate with a cell-repellent surface. After 1h plates are placed on an orbital shaker for the entire culture. Spheroid formation is already seen after 24h. Here a spheroid is shown at 5 days of culture and is stained for Lyve1(LSEC), Desmin (HSC) and DAPI (nucleus). References:
|
|
Project Lead: Early Stage Researcher:
|
During my bachelor Biomedical Sciences at the University of Utrecht (2012-2015) my interest started for research in disease modeling and liver research. With that motivation I started the master Biology of Disease at the University of Utrecht (2015-2017) where I focused on copper storage disease modelling in liver organoids at the Faculty of Veterinary Medicine at the lab of Prof. Bart Spee. For more insight into alternative sources for liver transplantation, I went to the Karolinska Institute to the lab of Prof. Stephen Strom were I worked on amnion epithelial stem cells as therapeutic cell source for the Alagille syndrome (rare genetic disorder that results in liver disease). After obtaining my Master’s degree in 2017, I started my PhD in 2018 at the LIVR cell biology research group of Prof. Leo van Grunsven on the role of liver sinusoidal endothelial cells in chronic liver diseases.
|
P