Several high profile research and review style publications have resulted from this action early on and the DeLIVER action and its EU funding have been acknowledged:
- V. Mönkemöller, et al., Primary rat LSECs preserve their characteristic phenotype after cryopreservation, Sci. Rep. 8, 14657 (2018) (Open Access)
- C. Øie, et al., New ways of looking at very small holes – using optical nanoscopy to visualize liver sinusoidal endothelial cell fenestrations, Nanophoton. 7, 575-596 (2018) (Open Access)
- L. Schermelleh, et al., Super-resolution microscopy demystified, Nat. Cell Biol. 21(1), 72-84 (2019)
During the action, a total of 23 papers have been published with acknowledgment of EU funding by the DeLIVER ITN, and approx. 10 more manuscripts are currently being prepared:
1 Butola, A. et al. Multimodal on-chip nanoscopy and quantitative phase imaging reveals the nanoscale morphology of liver sinusoidal endothelial cells. Proc Natl Acad Sci U S A 118 (2021). https://doi.org:10.1073/pnas.2115323118
2 Cauzzo, J., Jayakumar, N., Ahluwalia, B. S., Ahmad, A. & Skalko-Basnet, N. Characterization of Liposomes Using Quantitative Phase Microscopy (QPM). Pharmaceutics 13 (2021). https://doi.org:10.3390/pharmaceutics13050590
3 Cauzzo, J., Nystad, M., Holsaeter, A. M., Basnet, P. & Skalko-Basnet, N. Following the Fate of Dye-Containing Liposomes In Vitro. Int J Mol Sci 21 (2020). https://doi.org:10.3390/ijms21144847
4 Dellaquila, A., Le Bao, C., Letourneur, D. & Simon-Yarza, T. In Vitro Strategies to Vascularize 3D Physiologically Relevant Models. Adv Sci (Weinh) 8, e2100798 (2021). https://doi.org:10.1002/advs.202100798
5 Descloux, A. et al. High-speed multiplane structured illumination microscopy of living cells using an image-splitting prism. Nanophoton 9, 143–148 (2020). https://doi.org:https://doi.org/10.1515/nanoph-2019-0346
6 James, B. H. et al. The Contribution of Liver Sinusoidal Endothelial Cells to Clearance of Therapeutic Antibody. Front Physiol 12, 753833 (2021). https://doi.org:10.3389/fphys.2021.753833
7 Jayakumar, N., Ahmad, A., Mehta, D. S. & Ahluwalia, B. S. Sampling moire method: a tool for sensing quadratic phase distortion and its correction for accurate quantitative phase microscopy. Opt Express 28, 10062-10077 (2020). https://doi.org:10.1364/OE.383461
8 Jayakumar, N. et al. Multi-moded high-index contrast optical waveguide for super-contrast high-resolution label-free microscopy. Nanophoton 11, 3421-3436 (2022). https://doi.org:10.1515/nanoph-2022-0100
9 Jayakumar, N., Helle, O. I., Agarwal, K. & Ahluwalia, B. S. On-chip TIRF nanoscopy by applying Haar wavelet kernel analysis on intensity fluctuations induced by chip illumination. Opt Express 28, 35454-35468 (2020). https://doi.org:10.1364/OE.403804
10 Kaltschmidt, B. et al. Hepatic Vasculopathy and Regenerative Responses of the Liver in Fatal Cases of COVID-19. Clin Gastroenterol Hepatol 19, 1726-1729 e1723 (2021). https://doi.org:10.1016/j.cgh.2021.01.044
11 Kong, C. et al. Multiscale and Multimodal Optical Imaging of the Ultrastructure of Human Liver Biopsies. Front Physiol 12, 637136 (2021). https://doi.org:10.3389/fphys.2021.637136
12 Kong, C. et al. High-contrast, fast chemical imaging by coherent Raman scattering using a self-synchronized two-colour fibre laser. Light Sci Appl 9, 25 (2020). https://doi.org:10.1038/s41377-020-0259-2
13 Lalor, P. F., Huser, T. & van Grunsven, L. A. Editorial: Roles of Liver Sinusoidal Endothelial Cells in Liver Homeostasis and Disease. Front Physiol 13, 869473 (2022). https://doi.org:10.3389/fphys.2022.869473
14 Markwirth, A. et al. Video-rate multi-color structured illumination microscopy with simultaneous real-time reconstruction. Nat Commun 10, 4315 (2019). https://doi.org:10.1038/s41467-019-12165-x
15 McMillan, A. H. et al. Rapid Fabrication of Membrane-Integrated Thermoplastic Elastomer Microfluidic Devices. Micromachines (Basel) 11 (2020). https://doi.org:10.3390/mi11080731
16 Miron, E. et al. Chromatin arranges in chains of mesoscale domains with nanoscale functional topography independent of cohesin. Sci Adv 6 (2020). https://doi.org:10.1126/sciadv.aba8811
17 Opstad, I. S. et al. Fluorescence fluctuation-based super-resolution microscopy using multimodal waveguided illumination. Opt Express 29, 23368-23380 (2021). https://doi.org:10.1364/OE.423809
18 Pilger, C. et al. Super-resolution fluorescence microscopy by line-scanning with an unmodified two-photon microscope. Philos Trans A Math Phys Eng Sci 379, 20200300 (2021). https://doi.org:10.1098/rsta.2020.0300
19 Pospisil, J., Wiebusch, G., Fliegel, K., Klima, M. & Huser, T. Highly compact and cost-effective 2-beam super-resolution structured illumination microscope based on all-fiber optic components. Opt Express 29, 11833-11844 (2021). https://doi.org:10.1364/OE.420592
20 Szafranska, K. et al. Quantitative analysis methods for studying fenestrations in liver sinusoidal endothelial cells. A comparative study. Micron 150, 103121 (2021). https://doi.org:10.1016/j.micron.2021.103121
21 Szafranska, K., Kruse, L. D., Holte, C. F., McCourt, P. & Zapotoczny, B. The wHole Story About Fenestrations in LSEC. Front Physiol 12, 735573 (2021). https://doi.org:10.3389/fphys.2021.735573
22 Szafranska, K. et al. From fixed-dried to wet-fixed to live - comparative super-resolution microscopy of liver sinusoidal endothelial cell fenestrations. Nanophoton 11, 2253-2270 (2022). https://doi.org:10.1515/nanoph-2021-0818
23 Van den Eynde, R. et al. Quantitative comparison of camera technologies for cost-effective super-resolution optical fluctuation imaging (SOFI). J Phys-Photonics 1 (2019). https://doi.org:10.1088/2515-7647/ab36ae
News
1st Network Meeting - a big success!
The first full Network Meeting of the DeLIVER consortium took place on a Hurtigruten ship. All supervisors and ESRs assembled in Tromso, Norway, one of our main beneficiary sites, before boarding the ship for a 3 1/2 day meeting. This setting provided an excellent framework for all ESRs, as well as their supervisors, to connect and form a tight network.
All supervisors were asked to briefly present the research activities of their labs as part of the introduction, followed by all ESRs giving talks about their background and their projects as part of the action.
In addition, two renowned experts provided extended lectures to the consortium:
Prof. David LeCouteur (Scientific Director of the Ageing and Alzheimer's Institute at Concord Hospital, Sydney, Australia) from the DeLIVER partner site at the University of Sydney, Australia, gave an introductory lecture on liver sinusoidal endothelial cell (LSEC) biology and their role in ageing. LSEC and their unique morphology and properties are the main biological target of this action.
Prof. Friedemann Kiefer (one of the Directors of the European Institute of Molecular Imaging in Münster, Germany) from the University Hospital Münster, Germany, gave an introductory lecture on intravital microscopy and the challenges arising from intravital imaging, a topic that will become of great importance for the latter half of this action.
In addition, the DeLIVER project manager Dr. Mark Schüttpelz gave a scientific skills course in the form of a general introduction to superresolution microscopy, and the project coordinator, Prof. Thomas Huser, gave a soft skills course on Responsible Conduct in Research, where issues such as initiating and maintaining collaborations, dispute resolution, publications, and scientific misconduct were discussed with the ESRs.



Other Recent News:
Coming up: MidTerm Check Meeting in Brussels, April 29, 2019
Other events:
- Super-resolution optical fluctuation imaging (SOFI) workshop, ZiF plenary hall, Bielefeld, August 28-30, 2019
- 2nd Network Meeting, Birmingham, United Kingdom, October 2019
- ICON Europe 2020, Jena, Germany, 2020 (several DeLIVER PIs are co-organizers)
 |
Universität Bielefeld, Germany |
 |
Universitetet i Tromsø, Norway |
 |
University of Oxford, UK |
 |
University of Gothenburg, Sweden |
 |
University of Birmingham, UK |
 |
Vrije Universiteit Brussel, Belgium |
 |
LaVision BioTec, Germany |
 |
Cherry Biotech, France |
 |
Elvesys, France |
Please see here.
OPEN POSITIONS
PhD in Physics
The Department of Physics at the University of Gothenburg is located on the main campus of Chalmers University of Technology in Gothenburg, with a total of some 80 employees. The communication routes are good both nationally and internationally. The researching focuses within the fields of Atom- and Molecular Physics, Condensed Matter Physics and Spintronics, and Complex Systems and Biophysics and is performed in an international environment with extensive national and international collaborations. The education programs at the Department include Bachelor- and Master programs in Physics, physics teacher-training programs, as well as outreach courses in Physics aimed for the general public. Gothenburg Physics Centre is a close collaboration with four other departments within the Faculty of Science and Chalmers University of Technology creates an innovative environment for all researchers and students at the department.
For further information about the Department of Physics, please visit our website at www.physics.gu.se/english. More information about Gothenburg Physics Centre can be found at www.chalmers.se/en/centres/gpc/Pages/default.aspx.
The research will be conducted in an interdisciplinary research group at the University of Gothenburg, led by Caroline Beck Adiels (cell biologist) and Mattias Goksör (physicist) in close collaboration with a neighbouring group led by Giovanni Volpe. By means of optical and microfluidic technology, the group seeks to answer different research questions originating in biology and life science (http://www.physics.gu.se/english/research/biological-physics). The research group is now hiring a PhD student that will be involved in the EU consortium DeLiver.
DeLiver is a Marie Sklodowska-Curie Innovative Training Network (ITN) that include 6 academic and 3 industrial beneficiaries, represented in 6 European countries. This multidisciplinary collaboration combines research within optical physics and biomedicine with the aim of developing novel technologies to perform structural and functional studies (on nano-scale) of the pores of endothelial cells. In mammals, these pores constitute the fundamental barrier between the blood circulation and vital organs. (More information can be found here: http://www.deliver-itn.eu).
DeLIVER is an Innovative Training Network (ITN) funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 766181. The main purpose of DeLIVER is the training of 14 Early Stage Researchers (ESRs) in state of the art biomedical research and imaging methods to further our understanding of the structure and function of cells critical to liver function and healthy ageing. This network comprises a collaborative activity of 6 universities and 3 small and medium sized enterprises across Europe with additional partners from across the world.
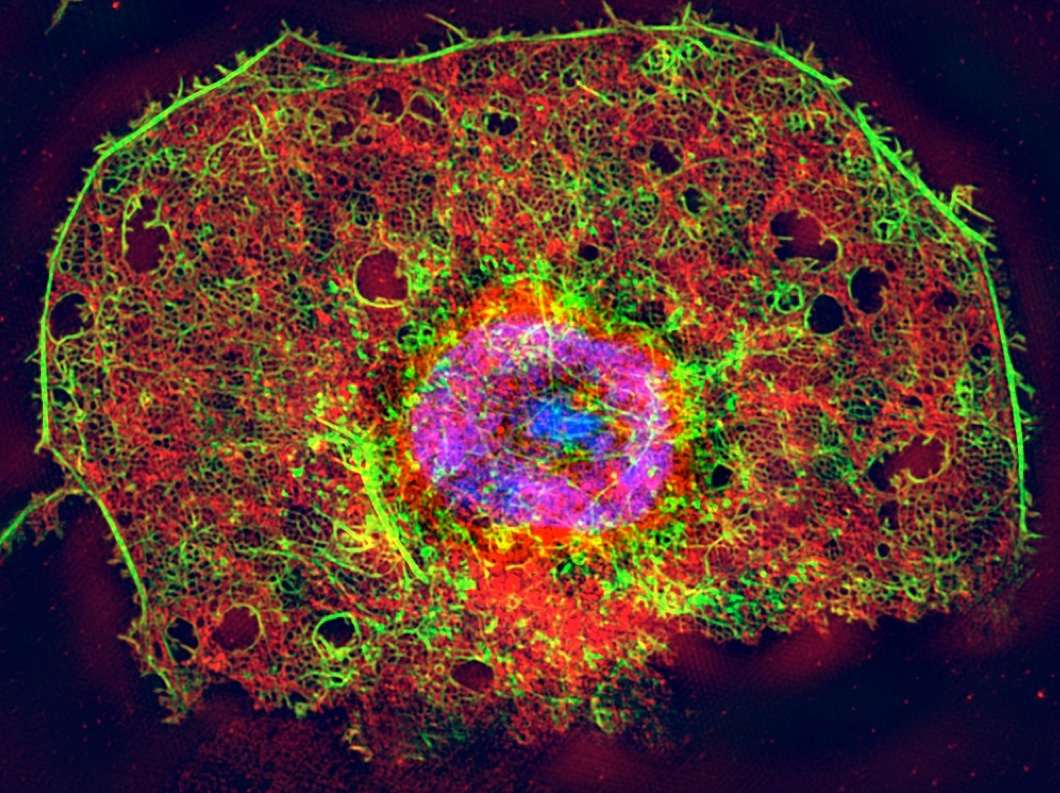 Structured Illumination Microscope (SIM) image
Structured Illumination Microscope (SIM) image
of a triply stained liver sinusoidal endothelial cell (LSEC)
The liver contains a large number of very fine capillaries (the sinusoids), which are lined by endothelial cells. In the liver, these endothelial cells are called liver sinousoidal endothelial cells (LSEC) and the contain thousands of nanosized pores that enable the clearance of molecules and small particles from the blood. The size of these nanopores is well below the optical diffraction limit and consequently, very little is known about the extremely important physiological function of these unique structures and their role in the transfer and/or clearance of metabolites and pharmaceuticals to vital organs. LSEC are also responsible for the removal and degradation of pharmaceuticals, virus and waste macromolecules, making them a highly relevant type of cell for the study of pharmacological drug uptake. Because of the issues mentioned above, however, most pharmaceutical companies currently cannot assess the effect of drugs on these cells.
Novel optical microscopy tools have become available in last few years that now enable for the first time the microscopic study of living cells on the nanoscale and their function.
The key research objectives of DeLIVER are:
- To develop novel high-speed super-resolution optical technologies that transcend the 200nm optical diffraction barrier
- To develop open-access tools for super-resolution optical image reconstruction and blue-prints for high-speed, real-time image reconstruction.
- To extend optical nanoscopy methods to 3D cell culture and in situ tissue studies.
- To obtain mechanistic descriptions of small molecule/pharmaceutical interactions with living LSEC, with liver tissue, and the effect of ageing on this process.
DeLIVER will train a new generation of Early Stage Researchers (ESRs) in the development and application of newly developed high speed and high resolution imaging tools in biomedical research. ESRs will be cross-pollinated with concepts and skills in physics and biomedicine, in particular in super-resolution optical imaging (a.k.a. optical nanoscopy), analytical image reconstruction, and optical micro-manipulation methods. These skills are applied to reveal for the first time the function and dynamics of nanosized pores in endothelial cells (EC) that present the main barrier between the blood and vital organs for human physiology, such as the liver, brain, kidneys, and the eyes. Excellent training in new scientific and complementary skills, combined with international and intersectoral work experience, will instil an innovative, creative and entrepreneurial mind-set in DeLIVER's ESRs, maximising economic benefits based on scientific discoveries. These specialised, highly trained, and mobile ESRs will have greatly enhanced career prospects. The training in novel physical methods with highly relevant experience in the biomedical sciences will allow them to confidently navigate at the interface of academic, clinical and private sector research.
Recent Publications
Select recent papers from the DeLIVER consortium:
- L. Schermelleh, et al., Super-resolution microscopy demystified, Nat. Cell Biol. 21(1), 72-84 (2019)
- C. Øie, et al., New ways of looking at very small holes – using optical nanoscopy to visualize liver sinusoidal endothelial cell fenestrations, Nanophoton.7, 575-596 (2018)
- V. Mönkemöller, et al., Primary rat LSECs preserve their characteristic phenotype after cryopreservation, Sci. Rep.8, 14657 (2018)
Recent News
Coming up: MidTerm Check Meeting in Brussels, April 29, 2019
Other events: - Super-resolution optical fluctuation imaging (SOFI) workshop, ZiF plenary hall, Bielefeld, August 28-30, 2019
- 2nd Network Meeting, Birmingham, United Kingdom, October 2019
- ICON Europe 2020, Jena, Germany, 2020 (several DeLIVER PIs are co-organizers)

