|
Development of a waveguide- and microfluidics-based integrated optical nanoscopy platform |
|
Project 2 - Nikhil Jayakumar University of Tromsø - The Arctic University of Norway |
|
Objectives: (1) Development of an integrated optics platform for real-time nanoscopic study of LSEC dynamics based on waveguide-SR-SIM (2) Increase field-of-view for single molecule localization microscopy to 200 µm x 100 µm based on waveguide excitation, implement nonlinear SIM approaches (3) demonstrate waveguide-integrated nanoscopy with microfluidic delivery for the rapid imaging of small molecule-LSEC interactions |
|
Expected Results: (1) Waveguide nanoscopy operational and imaging of fixed cells completed (90 nm, 20 frames/s) (2) field of view of waveguide nanoscopy increased to 200 µm x 100 µm (3) imaging of interactions of small molecules and nanoparticles with LSECs in vitro demonstrated |
|
Project Lead: Early Stage Researcher:
|
Nikhil Jayakumar was born in Kerala, India. He completed his Bachelors degree in Electrical and Electronics engineering from the University of Kerala and then worked under different verticals before moving to Jena, Germany in 2015. In Jena he graduated with a Masters degree in Photonics in 2017 and continued working as a scientific assistant before moving to UiT, Tromso for his Ph.D. He started his work in November 2018 under the supervision of Assoc. Prof. Dr. Balpreet Singh Ahluwalia and the scope of his work includes super-resolution imaging using waveguides and quantitative phase microscopy.
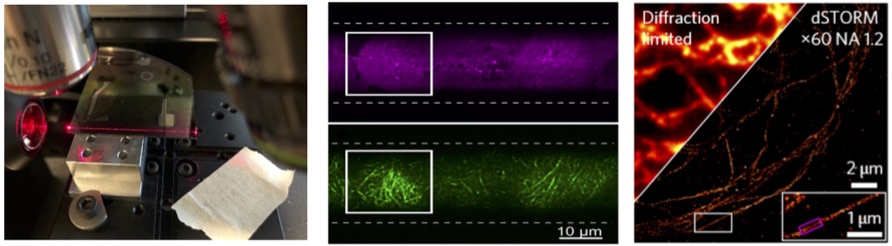
Nikhil Jayakumar's project will involve chip-based optical nanoscopy and quantitative phase microscopy. Chip-based optical nanoscopy: Our group have recently developed photonic chip based optical microscopy and nanoscopy methodologies [1, 2]. The use of a waveguide chip has enabled miniaturization of the optical setup by decoupling the excitation and collection paths (see Fig. 1a, below). The sample to be imaged is placed on top of the waveguide core area and the evanescent field generated due to total internal reflection at the core-sample interface illuminates. Due to evanescent field excitation only, a thin portion of the sample (a few hundred nm) is illuminated providing a very high signal-to-noise ratio TIRF images as shown in Fig. 1b. By using waveguide made of high refractive index contrast and by using thin waveguides the intensity of the evanescent field can be enhanced enabling blinking of fluorophores and the demonstration of on-chip optical nanoscopy using dSTORM principles as shown in Fig. 1(c) [2].The field of view (FOV) of the chip-based optical nanoscopy can be made large and we have recently reported optical resolution of 70-75 nm over FOV of 500 x 500 μm2 [3]. Nikhil Jayakumar's project will further work upon this novel idea of high throughput chip-based nanoscopy. Currently dSTORM imaging is limited by temporal resolution (takes approximately 30 minutes), as a large number of images (more than 20000 images) is required for a faithful reconstruction of the image. We intend to bring down this temporal resolution to a few seconds by employing intensity-fluctuation based algorithms such as MUSICAL, SOFI, ESI etc. which will facilitate high throughput imaging of liver sinusoidal endothelial cells. Another task is to build a portable photonic- chip based optical nanoscope and distribute within DeLIVER network.
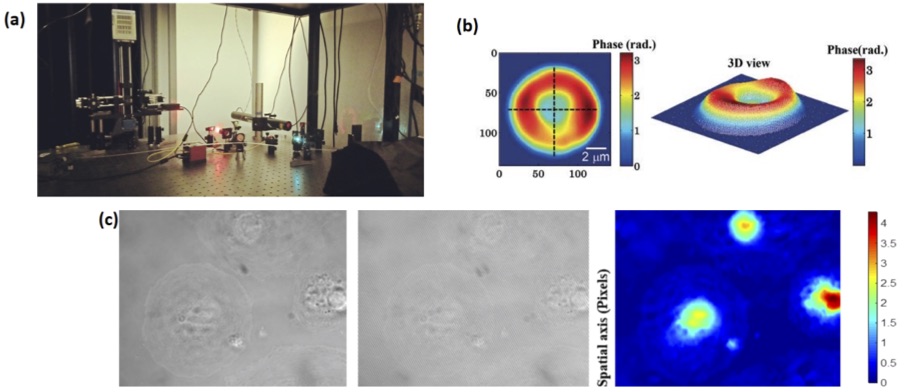
Figure 1: (a) Silicon nitride waveguide on a chip guiding light at 660 nm (b) TIRF image of stained MCC13 cells illuminated using a 25 μm wide silicon nitride waveguide (figure taken from Ref. 2) (c) Immuno-stained tubulin in LSEC under diffraction-limited imaging and d-STORM on waveguide based imaging (figure taken from Ref. 1) Quantitative phase microscopy: Biologists prefer to study cells living in their natural environment. However, most optical microscopic techniques rely on labeling the sample which is then no longer a natural state of the cell. And that is where quantitative phase microscopy (QPM) becomes useful. QPM provides a non-invasive non-contact imaging technique which can provide quantitative information like refractive index, thickness of the cell etc. It is known that when light passes through a sample that is weakly absorbing, which is the case for most cells, its phase changes. This change in phase contains information about the refractive index and thickness of the cell. The beauty of QPM is that to decode the information about the cell structure, the light beam which contains information about the cell needs to interfere with a reference beam, from which the relative change in phase can be deduced thereby ensuring label-free imaging. As part of this Ph.D. work, considerable emphasis will be laid on developing new techniques using the principles of QPM and improve the transversal and longitudinal resolution of QPM. By increasing the spatial resolution and temporal sensitivity of the novel quantitative phase microscopy, we aim to perform label-free quantitative imaging of the nanoscale fenestration present on the cell membrane of the LSECs. To enhance the spatial resolution of QPM, we will explore novel oblique angle illumination and Fourier ptychography imaging methodologies. And for enhancing the temporal sensitivity we will work with a common path approach. Fig. 2a shows the newly developed QPM at UiT and the results obtained from red blood cells (Fig. 2b) and initial results obtained from LSECs (Fig. 2c).
Figure 2: (a) Quantitative Phase Microscopy setup at UiT, a Linnik interferometer is used for the generation of interference fringes (b) 2D and 3D images of Red Blood Cells phase informatin obtained using QPM (figure taken from Ref. 4) (c) Bright field image of LSEC (A5), interferometric image of the same location and the reconstructed phase map of the similar cell. Bar represents the phase map “𝜙”in radian References: [1] - Diekmann et et.al. Chip-based wide field-of-view nanoscopy, Nat. Photonics 2017 doi: 10.1038/nphoton.2017.55 [2] - Tinguely, et.al. Silicon nitride waveguide platform for fluorescence microscopy of living cells, Opt. Express 2017; Volume 25 (22). doi:10.1364/OE.25.027678 [3] - Helle, et.al., Nanoscopy-on-a-chip:super-resolution imaging on the millimeter scale, Opt. Express 2019; Volume 27 (5). doi.org/10.1364/OE.27.006700 [4] - Ahmad, et.al., Quantitative phase microscopy of red blood cells during planar trapping and propulsion, Lab on a Chip 2018; Volume 18 (19). doi: 10.1039/c8lc00356d |